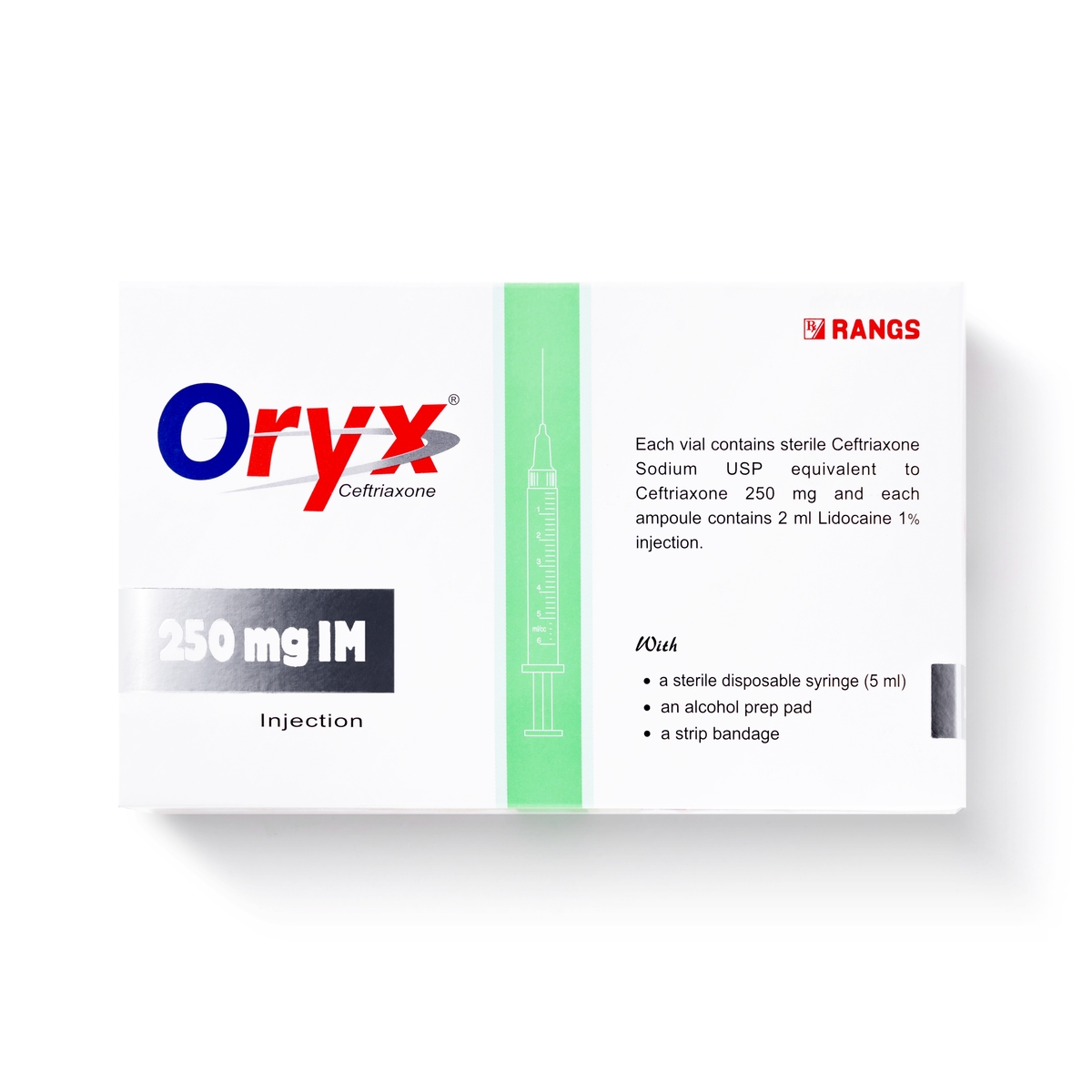
Oryx 250mg IM injection
Ceftriaxone Sodium 250 mg IM
Product Key Facts
Anti-infectives
Medications used to combat bacterial infections and inhibit the growth of bacteria.
Fighting Bacterial Infections
Fighting bacterial infections involves the use of antibiotics, which are medications designed to target and eliminate bacteria. The choice of antibiotic depends on the type of bacteria causing the infection. It's crucial to complete the full course of prescribed antibiotics, even if symptoms improve, to prevent antibiotic resistance.
Details at a Glance
| Product Code | : | 1432 |
| Pack Size | : | 1's |
| MRP | : | ৳ 100 |
Specifications
Oryx® 250 mg IM/IV Injection: Each vial contains dry substance equivalent to 250 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) accompanied by a solvent ampoule of 2 ml Lidocaine HCI USP 1% Injection for IM injection or 5 ml Water for Injection USP for IV injection.
Oryx® 500 mg IM/IV Injection: Each vial contains dry substance equivalent to 500 mg Ceftriaxone (as sterile Ceftriaxone Sodium USP) accompanied by a solvent ampoule of 2 ml Lidocaine HCI USP 1% Injection for IM injection or 5 ml Water for Injection USP for IV injection.
Oryx® 1 g IM/IV Injection: Each vial contains dry substance equivalent to 1 g Ceftriaxone (as sterile Ceftriaxone Sodium USP) accompanied by a solvent ampoule of 3.5 ml Lidocaine HCI USP 1% Injection for IM injection or 10 ml Water for Injection USP for IV injection.
Oryx® 2 g IV Injection: Each vial contains dry substance equivalent to 2 g Ceftriaxone (as sterile Ceftriaxone Sodium USP) and each of two ampoules contains 10 ml Water for Injection USP for IV injection.
Oryx® is indicated for the treatment of the following infections when caused by susceptible organisms: Lower respiratory tract infections, Acute bacterial otitis media, Skin and skin-structure infections, Urinary tract infections (complicated and uncomplicated), Uncomplicated gonorrhea (cervical/urethral and rectal), Pelvic inflammatory disease, Bacterial septicemia, Bone and joint infections, Intra-abdominal infections, Meningitis and in Surgical prophylaxis.
Oryx® can be administered either intravenously or intramuscularly. Dosage and mode of administration should be determined by severity of infection, susceptibility of causative organisms and the patient's condition. Under most circumstances, once-daily dose or in the specified indications, a single dose will give satisfactory therapeutic results. Adults and children over 12 years: The usual dosage is 1-2 g of Oryx® once daily (every 24 hours). In severe cases or in infections caused by moderately sensitive organisms, the dosage may be raised to 4 g once daily. Neonates, infants and children up to 12 years: The following dosage schedules are recommended for once daily administration. Neonates (up to 14 days): A daily dose of 20-50 mg/kg bodyweight, not to exceed 50 mg/kg, on account of the immaturity of the infant's enzyme systems. It is not necessary to differentiate between premature and term infants. Infants and children (15 days to 12 years): A daily dose of 20-80 mg/kg. For children with bodyweights of 50 kg or more, the usual adult dosage should be used. Intravenous doses of > 50 mg/kg body weight should be given by infusion over at least 30 minutes. Elderly patients: The dosages recommended for adults require no modification in case of geriatric patients. Duration of therapy: Oryx® therapy should be continued for at least 2 days after the signs and symptoms of infection have disappeared. The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required. When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days. Combination therapy: Synergy between Ceftriaxone and aminoglycosides has been demonstrated with many gram-negative bacilli under experimental conditions. Although enhanced activity of such combinations is not always predictable, it should be considered in severe and life threatening infections due to microorganisms such as Pseudomonas aeruginosa. Because of physical incompatibility, the two drugs must be administered separately at the recommended dosages. Meningitis: In bacterial meningitis in infants and children, treatment begins with doses of 100 mg/kg (not to exceed 4 g) once daily. As soon as the causative organism has been identified and its sensitivity determined, the dosage can be reduced accordingly. Best results have been found with the following duration of therapy: Neisseria meningitidis-4 days, Haemophilus influenzae-6 days, Streptococcus pneumoniae-7 days, Susceptible Enterobacteriaceae-10 to 14 days. Gonorrhoea (penicillinase - producing and nonpenicillinase - producing strains): a single IM dose of 250 mg Oryx® is recommended. Perioperative prophylaxis: A single dose of 1-2 g depending on the risk of infection of 30-90 minutes prior to surgery. In colorectal surgery, administration of Oryx® with or without a 5-nitroimidazole, e.g. Ornidazole, has been proven effective. Impaired renal and hepatic function: In patients with impaired renal function, there is no need to reduce the dosage of Oryx® provided hepatic function is intact. Only in cases of preterminal renal failure (creatinine clearance <10 ml/min) should the Oryx® dosage not exceed 2 g daily. In patients with liver damage, there is no need for the dosage to be reduced provided renal function is intact. In patients with both severe renal and hepatic dysfunction, the plasma concentrations of Ceftriaxone should be determined at regular intervals and, if necessary, the dose should be adjusted. In patients undergoing dialysis, no additional supplementary dosing is required following the dialysis. Plasma concentrations should, however, be monitored, to determine whether dosage adjustments are necessary, since the elimination rate in these patients may be altered.